Titanium dioxide and zinc oxide are often categorised as “physical” sunscreens, whereas every other sunscreen used is considered a “chemical” sunscreen.
| “Physical” Sunscreens | “Chemical” Sunscreens |
|---|---|
| Zinc Oxide Titanium Dioxide |
Octocrylene Avobenzone Octinoxate Octisalate Oxybenzone Homosalate Mexoryl SX Mexoryl XL Tinosorb S Tinosorb M … |
You’ll often find different rules and advice for using “physical” and “chemical” sunscreens. One dermatologist says that you need to apply less physical sunscreen compared to a chemical sunscreen. There’s also the belief that “physical” sunscreens provide protection instantly, don’t absorb into the skin, don’t degrade in the sun, and don’t need reapplication.
These are myths and are not backed by research or chemical knowledge. By following these rules (or myths) you’re not using your sunscreen to its greatest effect!
“Physical” vs. “Chemical”
Dividing sunscreens into “physical” and “chemical” isn’t the best way to do it. These two categories overlap completely. If we were to draw a Venn diagram of the two groups, it’d look like this
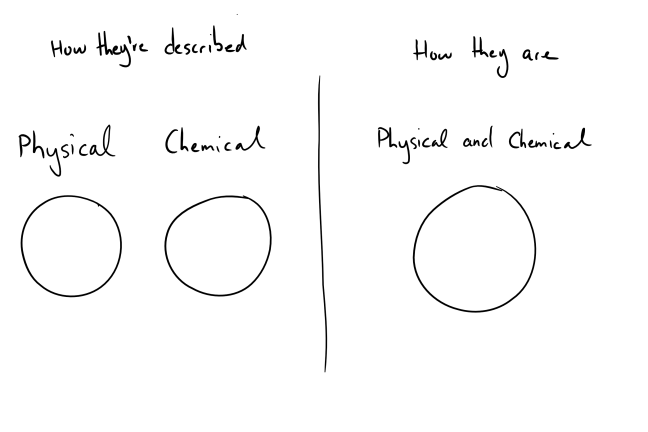
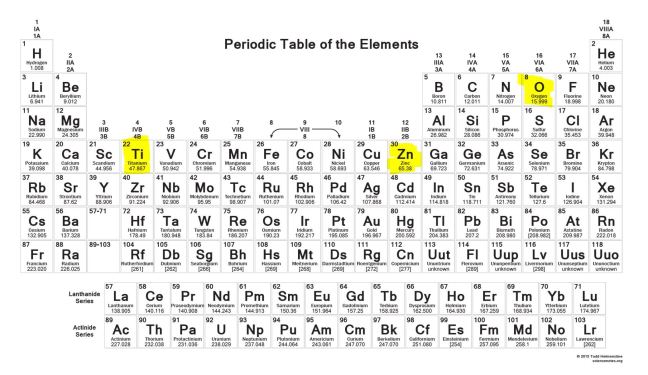
Chemicals are physical – they have a mass and take up space. On the other end, the “physical” sunscreens titanium dioxide and zinc oxide are chemicals, you can find the elements titanium and zinc on the periodic table.
It’s sometimes explained that titanium dioxide and zinc oxide are suspensions of particles, they don’t dissolve or form solutions like chemical sunscreens. This is true and their even distribution in the sunscreen formula and on the skin is very important – poor distribution can greatly reduce how much UV protection titanium dioxide or zinc oxide can provide on the skin.
However, there are caveats, sunscreens like Tinosorb M (INCI: Methylene Bis-Benzotriazolyl Tetramethylbutylphenol) also exist as particle suspensions – not solutions. Tinosorb M comes as a very fine suspension of particles in water. So, if you were to draw the line based on that you’d have to include Tinosorb M, a “chemical” sunscreen with the “physical” sunscreens.
What does differentiate titanium dioxide and zinc oxide then? Well, they’re both metal oxides or metals combined with oxygen. Metal oxide sunscreen doesn’t have the same ring to it, but there is another way to describe them.
Inorganic vs. Organic
In marketing, organic is a label that describes how something is produced – often with a safe-list of chemical treatments and approved practices.
In chemistry, organic means the chemistry of compounds that contain carbon. Titanium dioxide and zinc oxide don’t contain carbon. They’re made up of metal and oxygen and classified as inorganic.
Marking the categories as organic and inorganic makes more sense because all of the sunscreen chemicals used contain carbon, except for titanium dioxide and zinc oxide.
| Sunscreen | Chemical Formula | Composition |
|---|---|---|
| Zinc Oxide | ZnO | 1 Zinc + 1 Oxygen |
| Titanium Dioxide | TiO2 | 1 Titanium + 2 Oxygens |
| Octocrylene | C24H27NO2 | 24 Carbons + 27 Hydrogens + 1 Nitrogen + 2 Oxygens |
| Avobenzone | C20H22O3 | 20 Carbons + 22 Hydrogens + 3 Oxygens |
| Octinoxate | C18H26O3 | 18 Carbons + 26 Hydrogens + 3 Oxygens |
Organic and inorganic is also a useful way to categorise sunscreens because the way that the carbon atoms are linked up in organic sunscreens is why they absorb UV energy. If you look at the chemical structure of an organic sunscreen like avobenzone you’ll see that they have single bonds alternated with double bonds.

This alternation or conjugation of the single and double bonds allows the molecule to absorb energy along the electromagnetic spectrum. The amount of conjugation determines which part of the electromagnetic spectrum they absorb, whether that be in the visible spectrum to produce a colour, or in the ultraviolet spectrum to protect our skin from UV.

Inorganic and organic neatly divide the two sunscreen types and are also descriptive. I know most companies won’t want to confuse their customers by labelling their 80% organic-certified sunscreen product with titanium dioxide as inorganic, but at least as sunscreen shoppers we can understand the difference!
Myths about using Inorganic vs Organic Sunscreens
“Inorganic sunscreen and organic sunscreens work differently”
Mostly Myth! It’s often said that inorganic sunscreens (titanium dioxide and zinc oxide) reflect UV off of the skin and organic sunscreens absorb UV and convert it into heat. In reality, for most of the UV spectrum they work very similarly.
Organic sunscreens absorb UV because of the way the bonds between their carbon molecules are arranged. The number of bonds between the carbon atoms in the sunscreen molecules and their conjugated arrangement give sunscreens their absorptive properties in the UV region of the electromagnetic spectrum. Remember that conjugated means alternating single and double bonds!
The energy from UV light promotes electrons in the conjugated carbon bonds of organic sunscreen molecules from a lower energy state to a higher energy excited state. The excited electrons in the bonds then relax or release the absorbed energy by stretching, vibrating, or bending – this turns that energy into heat.
In some cases, the organic sunscreen chemical can’t relax and release the absorbed energy by bending, stretching, or vibrating and the absorbed energy causes a change in its structure. This is what happens with avobenzone, it absorbs the UV energy and instead of relaxing, it changes its structure – and this new structure formed from avobenzone doesn’t absorb UV energy as well. As more and more avobenzone molecules’ structures change, the less UV energy is absorbed by the sunscreen formula. Some of the new structures formed from avobenzone are also more irritating and sensitising to the skin. Photo-stabilizers prevent this from happening by absorbing the energy from excited avobenzone and releasing it before its structure can change.
Inorganic sunscreens work very similarly – even though their structure is different from organic sunscreens. Metal oxides, like titanium dioxide and zinc oxide, have solid structures made of alternating sheets of metal and oxygen atoms. The principle behind the UV protection is exactly the same as organic sunscreens. Instead of the arrangement and amount of carbon bonds, the particle size of the titanium dioxide or zinc oxide determines which parts of the electromagnetic spectrum it absorbs.
There is a strong belief that these inorganic metal oxide sunscreens act by reflecting UV light instead of absorbing it, but this isn’t the complete story. UV light is divided into UVB and UVA. UVB is between 280 to 315 or 320 nm and UVA is between 315 or 320 to 400 nm. Inorganic sunscreens predominately absorb in the UVB spectrum and reflect in the long UVA (above 360 nm) and visible spectrum. Only about 5% of UVB light is reflected by inorganic sunscreens and the remainder gets absorbed and converted – just like organic sunscreens.
The results of a measurement show how much energy is reflected by different types and sizes of titanium dioxide. The horizontal scale represents the electromagnetic spectrum with my yellow highlight marking the UV spectrum. The vertical scale represents how much of the energy is being reflected, the higher up on the chart – the greater the amount of reflection.

Between 250 nm and 350 nm titanium dioxide reflects less than 10% of the energy. Between 350 nm and 400 nm there is more reflection depending on the form of titanium dioxide and the particle size. The anatase form of titanium dioxide exhibits more reflection than the rutile form of titanium dioxide. These forms have to do with the way the titanium and oxygen atoms are arranged in the titanium dioxide. Sunscreens often use rutile titanium dioxide because they are safer and less reactive.
The same is seen with zinc oxide, with most of the reflection being above 350 nm. The rest of the UV spectrum is absorbed.

The high reflection above the UV spectrum (above 400 nm) into the visible light region of the electromagnetic spectrum is what causes the whitening effect and flashback when using inorganic sunscreens.
“You can use less of an inorganic sunscreen compared to an organic sunscreen”
Myth! All sunscreens are tested at the same density, which is 2 milligrams of sunscreen per square centimetre. That applies to inorganic, organic, spray, stick, lotion, wipes, etc.
If you want to get as close as possible to the protection on the label of the sunscreen product, you need to apply it at the same density it was tested at.
“Inorganic sunscreens sit on the skin. Organic sunscreens absorb into the skin”
Myth! Think of it this way, if we want to protect ourselves from the rain we need to hold the umbrella above our heads. Sunscreens work the same way, you want them to absorb the energy before they can reach our skin cells, particularly the living cells. The most effective way for this to be done is to have them on the surface of the skin in a continuous and even layer.
Both organic and inorganic sunscreen particles can penetrate into the upper layers of the skin. If and how much they penetrate is dependent on properties like their particle or molecular size as well as the overall sunscreen formula. This isn’t a desired effect and formulators work to reduce the amount that penetrates. Modern organic sunscreens often have larger molecular sizes, chemical and physical properties, or even coatings which make it more difficult for them to penetrate past the surface of the skin.
Keep in mind that skin penetration doesn’t mean that it’s causing harm to our bodies. There has to be a biological mechanism for it cause an effect. There is a lot current and ongoing research into this area, but we don’t have any strong answers yet.
“Inorganic sunscreens provide protection right away. Organic sunscreens need to activate on the skin”
Myth! Organic sunscreens and inorganic sunscreens absorb UV due to their electronic properties. There’s no activation or chemical reaction that occurs on the skin with organic sunscreens to create photoprotection.
We know this is true because we can measure how much UV is absorbed by an organic sunscreen off of the skin, like on a piece of clear plastic. Organic sunscreens will also prevent UV colour changing bracelets, beads, or stickers from changing colour.
Both inorganic and organic sunscreens will provide UV protection as soon as they’re placed on the skin. The reason why a wait time is part of the application instructions is to allow the sunscreen formula time to dry and form a film on the skin. This makes it harder for it to be wiped off and it also means it can dry to as even of a film on the skin as possible.
The more evenly distributed the sunscreen is on the skin, the more even the coverage and the greater the average protection. If we take 10 umbrellas and hold them over one person, that one person may remain very dry during a downpour but everyone else will get soaked – if we distribute the umbrellas evenly more people will remain dry. Photoprotection works the same way, it’s measured as an average – you don’t want some areas of the skin with more sunscreen and greater coverage at the expense of other areas with less sunscreen and less coverage.
“Inorganic sunscreens don’t need to be reapplied”
Myth! All sunscreens should be reapplied if you want to maintain photoprotection throughout the day. While it’s true that titanium dioxide and zinc oxide don’t change structure under normal UV radiation, that’s true for many organic sunscreens and sunscreen formulas as well.
The reason why reapplication is recommended is because we often don’t apply enough in the first place and it’s constantly being removed from our skin. Reapplication helps ensure that we have a minimum density of 2 milligrammes per square centimetre of sunscreen on our skin and that we maintain that density throughout the day.
We may not be conscious of removing our sunscreen, but touching our skin, putting on and taking off clothing, using our phones, sweating, eating…all these things will remove some of the sunscreen from our skin. Think about how the coverage of a foundation or lipstick changes throughout the day.
There is no clear answer as to when you should reapply your sunscreen. We all do different things throughout the day in regards to our skin, so the amount of sunscreen removed from the skin will differ from person-to-person and day-to-day. That’s why it’s difficult to have a single rule that will apply to everyone. Conclusions from studies vary in their recommendations for when and how often to reapply.
What you choose to do is up to you, but you should take into account how much UV you’re exposed to, how much you expect to be exposed to, and your activities. You should think about reapplying your sunscreen before going for a jog outdoors. Work in an office? Maybe reapply before you leave the office. What’s clear though is that you should definitely reapply after sweating, swimming, bathing, and abrasion (like laying on sand) – even if you are using a water-resistant sunscreen.
In the UK many sunscreens are marketed as ‘once-a-day’, but health organisations recommend disregarding that and still reapplying throughout the day.
I hope this post has helped you understand why calling some sunscreens “physical” and others “chemical” isn’t as descriptive as it could be, as well as why inorganic and organic sunscreens should be used the same way. Sunscreen is an important part of a skincare routine, and there’s a lot of conflicting advice on how to best use it. Understanding some principles will help you make sense of what is good advice and poor advice when it comes to sunscreen.
I’d also like to thank my friend Jonathon Moir for his help in editing this article.
