I originally wrote this for Sokoglam’s Klog, however, I think it’s still worth sharing and hopefully you find it informative and interesting!
Cleansers are one of the few times in skin care when we actually remove things from our
skin, and a good cleanser will remove things that we don’t want on our skin, while minimizing the removal of things we do want in our skin. Removing soil, makeup, sunscreens, sebum, and pathogenic microbes are beneficial to the health of our skin, but the chemicals we use to dissolve and remove those unwanted substances can also remove components from our skin that provide it protection and moisture.
How do cleansers work?
Cleansers work by dissolving and emulsifying things off of the skin that aren’t normally rinsed away by water. Water on its own can rinse away some soils, but it has a harder time rinsing away lipids – like the oils and waxes produced by our skin and added to the skin from products.

Modern cleansers employ surfactants, named because they are active on the surface (surface active agent) of where two things meet – in this case water and lipids. One end of the surfactant molecule dissolves into lipids more readily, and the other end dissolves into water more readily. When surfactants come into contact with lipids and embedded soils on the skin they lift it off the skin’s surface and because the surfactant can still partially dissolve in water, the whole mixture can be rinsed off with water.
Two aspects that contribute to a cleansers gentleness or harshness
Micelles
When we pour a tiny amount of surfactants into water, they’ll all sit on the surface of the water in a film, with their lipid dissolving ends sticking up into the air.
<
Hydrophobic means water-fearing, these are the lipid dissolving ends of the surfactant molecule. Hydrophilic means water-loving, these are the water dissolving ends.
Once there’s no more room on the surface, additional surfactants are pushed down into the water. Here they can begin to form bubble-like structures called micelles.
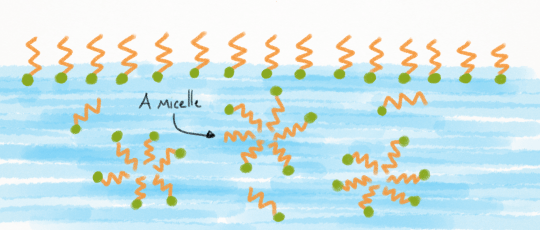
Now what, exactly, is a micelle? A micelle is a sphere, with the surfactants’ lipid dissolving ends pointed towards the center. This keeps them as far away from the water as possible. (Heard of micellar water? That’s a dilute solution of water with surfactant micelles in them)
The formation of micelles isn’t permanent though. These spheres are constantly rearranging and reforming, breaking into individual surfactant molecules and rejoining other micelles.
Surfactant molecules can also organize in ways other than spheres, such as the lipid bilayer seen in our skin.

Individual surfactant molecules are harsher to the skin than grouped surfactants in a micelle. The individual surfactant molecules’ smaller size allows them to penetrate past the outer layers of the skin and work their way in-between our skin lipids, which help retain moisture of the skin. Surfactants can also bind to proteins, enzymes, and cell membranes. This warps their structures, which can lead to inflammation. We see this inflammation as redness, and eventually scaling and drying of the skin.
Some individual surfactant molecules are so small, like sodium lauryl sulfate, that even their micelles are small enough to penetrate past the outer layers of skin. Sodium lauryl sulfate, on its own, is so good at causing irritation that it’s often used as an irritation control in skin studies.
By reducing the amount of free individual surfactants in a cleanser and by increasing the size of a micelle, we can make a gentler cleanser! This can even be done with a harsh surfactant, like sodium lauryl sulfate. Just because it’s on an ingredient list, doesn’t necessarily mean the cleanser is going to be harsh.
One way mixing surfactants can increase the micelle size is by being different sizes. The size difference of the different surfactants disorganizes the sphere shape of the micelle and increases the surface area, which creates a gentler cleanser.

There are other ways to increase the size of micelles and reduce free individual surfactants too. The most common way is to add proteins and polymers into the cleanser. These not only join in on the micelles’ structure – enlarging them, they can also bind free surfactants, so they’re less likely to bind to your skin’s components.
pH
The pH of a cleanser can have an impact on the harshness of a cleanser as well, but most often in an indirect way – especially with modern surfactants.
Our skin’s surface has acidic components on it that create a slightly acidic environment which keeps certain bacteria at bay. Each deeper layer of skin increases in pH, eventually reaching a less acidic pH closer to 7. This pH gradient has important functions, such as controlling the activity of enzymes in different layers of the skin.
Cleansers affect the skin in two ways, and the first is a chemical interaction. Acidic and alkaline chemicals interact and form new compounds which can alter the pH of the skin.
The second way skin’s pH is affected by a cleanser is simply by the removal of acidic components, like free fatty acids and others, from the skin. This is why almost any cleanser, including water, can temporarily raise the pH of the skin.
Healthy skin can replenish its pH to normal levels on its own, often in less than an hour. One of the ways skin maintains its acidic surface is through the activity of enzymes that are constantly breaking down lipids and other components of the skin and turning them into acids.
Using an acidic product after cleansing won’t restore the acidic components back to the skin, but it may help facilitate your skin’s process in creating new acidic components. In the same sense, adding alkaline substances to the skin may hinder the process.
When it comes to cleansers, this all just means that if we take two cleansers that are identical except for the pH, the acidic one is often (but not always) more gentle. pH adjusted cleansers often contain surfactant mixtures with larger micelles as well!
Putting it all together…
As a consumer it can be difficult to tell if a formulation is going to be gentle or not, especially based on the ingredients and a pH test. There are even more factors that determine the mildness of a cleanser and some of that is subjective.
If you’re looking at listed ingredients, decyl glucoside is considered a very mild surfactant. However, it removes almost as much lipid from the skin as sodium lauryl sulfate. It can be formulated to predominately remove lipids from the surface of the skin, which maintains its mildness, but it might also leave your skin feeling stripped, since there’s no lipids on the surface.
Some tips to identify gentle cleansers
Look for multiple surfactants! I personally look for surfactants like decyl glucoside, coco-glucoside, disodium cocoyl glutamate, disodium laureth sulfosuccinate, cocoyl methyl glucamide, sodium cocoyl isethionate, and lauryl lactyl lactate. Though the names can be hard to pronounce, they’re very mild to the skin on their own, reduce irritancy of harsher surfactants, and are bio-degradable.
Look for skin-moisturizing ingredients, like oils, butters, proteins, and amino acids.
Be cautious about high pH ingredients like sodium palmitate, sodium cocoate, and other saponified oils. These surfactants aren’t easily pH adjusted, as they form fatty acids in acidic solutions which are oily and less effective cleansers.
Keep in mind this list isn’t exhaustive!
How to use a cleanser gently
Use cool or cold water to clean your skin! This makes it less likely for lipids to be removed from within the skin. Hot water can also decrease the size of micelles and increase the amount of free surfactants, which leads to more irritation.
Use less cleanser! A cleanser doesn’t need to foam for it to work. The concentration of surfactants at which they start forming micelles is often very low. For example, for gentler surfactants like decyl glucoside it’s around 0.08%. Most cleansers contain between 15% to 60% surfactants.
Use something after! Even water will remove some lipids from the surface of the skin, so it’s helpful to put on a moisturizer. Those with oily skin might consider a watery serum or gel.
Those with very sensitive skin may consider using diluted cleansers, like micellar water or cleansing lotions. Using them with a cotton round assists in the removal of soil and grime. I’d still recommend rinsing your face after cleaning, though, because sometimes surfactants left on the skin can lead to irritation.



